News/Photo
Dunas Meeting Component 3
On 29 Jan 2026, JKT DUNAS component 3 on Access of Medicine was held in hybrid and chaired by Pn Zuhaini binti Mukrim, Director of Bahagian Amalan & Perkembangan.
- MOPI presented predatory pricing by MNC affecting local generics launching. Duopharma spent RM30M + RM2M in CAPEX and R&D on Trevive, but Norvartis drop prices of Glivec fr RM6000 to RM 1000. Similarly, Roche drop prices of Herceptin from RM6000 to RM3000. PhAMA mentioned the short timeframe of 5 to 6 years only to market in Malaysia before losing patent. Solution from A&P: to award tender to 2 products to get maximum benefits. MAPS mentioned one of the solutions is to shorten registration timeframe. NPRA responded Quest 5 and future AI evaluation will shorten the time of registration
- Draft formulary TCM has been approved in Oct 2025. Directive will be sent out soon
- Medicine shortage Google form implemented. It will be mandatory by 1 July 2026. Guideline on reporting of medicine shortage and discontinuation already uploaded to NPRA website
- A&P noticed many medicines increase their prices comparing from KPK approval to registered product. APHM cited no competition leads to that. MAPS mentioned the need to meet registration requirements like Zone 4B and BE pushed said drug prices higher. Consensus: quality and safety still the priority and registered drugs ensure regular availability of medicines
- No initiative yet to do Drug pricing control. Even setting ceiling price is deemed not feasible, as it will affect accessibility of Medicines
- Pharmacoeconomic framework is mandatory for listing into Drug formulary
- Guideline for submission of dossier for listing into the MOH Formulary to be implemented before Dec 2026
- To shorten timeline of accessibility of new medicine, PhAMA suggest doing concurrent registration and blue book listing process
- 8th NEML will be revised and published in 2026
- 15 generics granted priority review approval
- MH Nexus awarded with Quest 5 tender and SST signed on 2 Jan 2026
- Pool Procurement of Public and Private framework developed but deem this initiative not able to be carried out due to inability to guarantee benefits to the public
- Joint API stockpiles proposed instead by Malaysia Productivity Corporation
- Price transparenvy mechanism to display prices at private sector faced objection from Drs and Dental Associations. Court grants interim suspension to this initiative
- National Generics Medicines and Biosimilars framework developed in June 2025
- Objective is Affordability and accessibility of medicine with enhanced competition to manage price escalation of medicines
- 5 strategies, 15 Initiatives and 17 Indicators with 70.58% achieved.
Dunas Meeting Component 4
On 21 Jan 2026 9am to 10:30am, Ybrs En Mohd Zawawi bin Abdullah, Director of Enforcement division chaired the meeting on DUNAS (Dasar Ubat Negara) Component 4 on Quality use of medicines. Overall achievement is 87.76%
- Moving forward will invite Shopee, Lazada and Tik Tok to handover declaration on ToBAT (Tolak Ubat Tidah Sah) in conjunction with National Regulatory Conference in July 2026
-
Braille products currently not in tender specification. However, PRH can put braille as a value-added benefit during tender submission
-
Draft on Good Dispensing Practice on the way
-
Amendment on sales of drug act to be tabled to parliament in July 2026.
MyCEB and RSE Meeting with MAPS
On 12 Jan 2026 at 2pm, MAPS met up with personnel from MyCEB (Malaysia Convention & exhibition Bureau) and RSE (Reed Sinopharm Exhibitions) on possible collaboration, especially during the below events:
- tHIS (The Health Industry Series) for Asean participants dated 28-30 July 2026 at KLCC Convention Center. Organizer: RSE + APHM.
-
PharmChina dated 13-15 May 2026 Shanghai with 4500 distributors and 2000 exhibitors. The biggest exhibitions for pharmaceuticals in China
- China International Medical Equipment Fair dated 9-12 April 2026 at Shanghai with 5000 exhibitors.
We discussed on the challenges met with China manufacturers mainly in:
- Regulatory hurdles. (GMP inspections , Zone 4B, BE etc)
- Volume hurdles. (Expectation and batch size too big for Malaysia market)
Click for more info on tHIS 2026 and Reed Sinopharm.

TWG BE meeting
The meeting was chaired by Pn Rosliza as Timbalan Pengarah on 26th Nov 2025. There were two topics discussed:
1. MCP for Ibuprofen
2. Biowaiver for additional strength
Pharmaniaga Vendor Development Program
MAPS organized a dialogue with Pharmaniaga VDP with 3 of their team members on 26 Sept 2025 from 10.30am - 1.00pm. Discussions involved:
- Minimum Shelf life maintained at NLT68%
- Performance bond of 5%
- Reschedule of delivery due date
- Return goods
- Invoice & Payment
Presentation slides which include Pharmaniaga feedback has been sent to members via email on the same day.

ASEAN Consultative Meeting on ADSSR
On May 26th Members of the Association of Southeast Asian Nations (ASEAN) committed to ASEAN Drug Security and Self
Reliance, and the Framework on Regional Collaboration for ADSSR
On the back of this commitment, the ASEAN Consultative Meeting on ADSSR: Development of the Strategic Plan of Action & Implementation, was held on 23rd September 2025, at Zenith Hotel Putrajaya, Malaysia. MAPS President attended the meeting on the first day. The next 2 days were closed sessions among government staff during which the issues and challenges related to medicines security in the ASEAN region were shared and examined and the proposed plan of actions gathered from all Asean Member States and a comprehensive plan of action was to be developed.The situation analysis is available to download

Medicine Security Initiative Engagement Session
Back in 2022, the nation experienced medicine shortage for many products. Datuk Seri Dr. Dzulkefly bin Ahmad, who would later be appointed Health Minister called for a national "medicine security" strategy to be devised to prevent future drug shortages in Malaysia, given the country's current vulnerable position as a net importer of pharmaceutical products.
This engagement was held over 3 days from September 17 to 19th, 2025 at the Putrajaya International Convention Center. Inputs were collected from 5 groups that discussed on the topics of - Procurement & Supply Chain
- Regulatory
- Research & Development
- Manufacturing
- Foresight
All the inputs were presented to the Deputy Director General of Health (Pharmacy), Dr Azuana Ramli on the last day, representing the Secretary General of Health.

Ministry of Health Budget 2026 Dialogue
MAPS attended the session under the auspices of the Health Ministry at the Everly Putrajaya on Sep 4, 2025. The top management of the MOH was there to take suggestions from the huge crowd of stakeholders among industry, professional bodies, health ministry staff, patient bodies, NGO's, etc. The Minister of Health Datuk Seri Dr. Dzulkefly bin Ahmad was accompanied by the Secretary General Dato' Sri Suriani Binti Dato' Ahmad and Director General Datuk Dr. Mahathar Abd Wahab were available to address immediate questions. The Secretary General led the dialog.

Dr Choe Tong Seng Conferment as Fellow of MPS
Congratulation to Dr Choe Tong Seng,FMPS for his conferment as a "Fellow" by the Malaysian Pharmacists Society (MPS) at the National Pharmacists Convention 25 July 2025 in Marriot, Putra Jaya. It is a well deserved honour and recognition.
Below is the citation read out by Mr Jack Lim, the General Secretary of MPS:
Dr. Choe Tong Seng has been a respected figure in Malaysia's pharmaceutical industry for more than four decades.
He rose from the ranks to become a Regional Director for ASEAN in multinational companies and served as Executive Director of MAPS for over a decade.
A former President of PhAMA and Deputy President of MPS, he has been a consistent advocate for fair regulation, market integrity, and local industry resilience. He also mentors young pharmacists through leadership programmes.
Today's Fellowship recognises his strategic contributions to both industry and professional policy.

CPHI South East Asia 2025
On 17 July 2025, President Mr Lim Teng Chyuan presented on the topic "Strategies for pharmaceutical import and market access in Malaysia" to a hall full of potential investors into the country. As for those who are willing to work with MAPS, information has been circulated via email to all members.

International Healthcare Week 2025 KL official launch
International Healthcare Week (IHW) 2025 was held from July 16-18, 2025, at the Malaysia International Trade and Exhibition Centre (MITEC) in Kuala Lumpur. The event is Southeast Asia's premier healthcare event and encompasses five key exhibitions: CPHI South East Asia, WHX Kuala Lumpur, WHX Labs Kuala Lumpur, Medtec Southeast Asia, and HIMSS APAC. IHW 2025 aims to drive innovation, investment, and collaboration across the healthcare industry.
The Minister of Investment, Trade and Industry, YB Senator Datuk Seri Utama Haji Tengku Zafrul bin Tengku Abdul Aziz officiated the opening ceremony on the 16th July in the presence of delegates from the diplomatic community, various government agencies and industry. MAPS is one of the supporting organisations to this event, with the President attending the opening ceremony.

Dialogue with NPRA Number 1/2025
MAPS had a dialogue with NPRA on 20 Jun 2025 to discuss various regulatory issues, with representation by Mr Heng, the Chair of MAPS's Regulatory Committee supported by Ms Chong Siew Mei and several RA personnel from member companies. Minutes of Dialogue and Pre-dialogue with NPRA has already been circulated via email.
NPRA would like MAPS members to update email address in the Quest system and to ensure Good Submission Practice with quality dossiers. Take note that
- Quest 5 will allow auto product renewals.
- For NEML list and KPK Approved items, BE can be exempted with justifications [ eg. has been in the market for 10 years and no PV issues faced etc....]
- Additional indication for generics when innovators are not in the market: Will be accepted on case to case basis.
- Products under renewal and variation same time, to alert HOD to proceed for renewal first.
- Dissolution specifications to align with Bio batch data: However, in situation where product is unable to follow bio batch, can be considered acceptable if the proposed specification is the same as approved by the reference agency with assessment report as supporting documents. Dissolution limit based on monograph can be considered case to case basis.
- NPRA agreed to have a simplified evaluation process for second source. Pilot study will be conducted.
- 2 batches of in use stability data is required at the point of screening: If the SS batches are no longer available, other on-going SS batches can be considered.
- The pilot study to revise or minimize variation timeline is in progress and will complete by third quarter 2025.
- NPRA will consider reducing storage conditions to Zone II if product is not self administered and stored by patient with justification and proof that product is unstable at Zone IVb.
- NPRA collected our wish list for Quest 5 and will see that it meets users' requirements esp. on document upload size, multiple variations allowed etc.
- CoPP (Export only) to be accepted for registration: NPRA may accept on case to case basis especially products that are needed by the country.
- NPRA may accept WHO GMP certificate for non FRP route , only for WHO Pre-Q products only

Dialogue Mechanism on Implementation of Reporting of Medicines Disruption or Shortage or Discontinuation
A dialogue 2.0 on mechanism on implementation of reporting of medicines disruption or shortage or discontinuation in Malaysia has been held on 17 June 2025. This dialogue is chaired by YBrs. Pn Rosliza Binti Lajis. Crisis in 2022 involved 54 APIs and 1384 pharmaceutical products out of stock or facing drug shortage. DUNAS requires monitoring and mitigation of these disruption. Therefore, UPC has been conducted to gather feedback from all parties whereby 122 questions were raised. Proposed reporting time has been amended from 48 hours to 3 working days. To safeguard confidentiality and ensure reports are from authorized source, Company ID, Name and IC of active token holders are required during reporting. 1 Aug to 31 Dec 2025 Voluntary implementation. Starting 1 Jan 2026, Mandatory implementation. Guidelines and presentation slides have been circulated to members on 17 June 2025.
27th Meeting of the ACTD/ACTR Implementation Working Group
On 11 to 13 June 2025, MAPS attended as observer in both IWG & PPWG for Asean Technical working group. The meeting focused on the collaboration among ASEAN member states in implementing the ACTR and ACTD, with an emphasis on site-specific stability data.
Thailand officially transferred the chairmanship of the Implementation Working Group to Indonesia, with Tri Asti Isnariani expressing appreciation for the opportunity to lead. The ASEAN Secretariat provided updates on the outcomes of the PPWG meeting, which included the development of Q&A documents for site-specific stability data requirements. Thailand presented findings from the 26th IWG meeting, emphasizing the need for a review of current guidelines and a survey among member states regarding their practices.
A significant discussion centered on Vietnam's new pharmaceutical circular, which replaces Circular 08. The representative from Vietnam confirmed that the new Circular 12 addresses previous concerns by eliminating the requirement for a Certificate of Pharmaceutical Product from stringent regulatory authorities for new pharmaceutical products. Tri Asti Isnariani requested further clarification on the authentication of CPPs, to which Vietnam responded that only CPPs from the manufacturing country are now required. Indonesia expressed its commitment to conducting a survey on the implementation of site-specific stability and will share the results with the lead country of the AVG for further action, while Thailand highlighted the need for enhanced understanding and training on SSS implementation among member countries.
Zhongrui Pharmaceutical Business School China visit to MAPS
On 11 June 2025, 15 team members from Zhongrui business school China, representing over 2000 manufacturers in China visited MAPS Secretariat and had an extensive discussion on how to collaborate to bring in China products to meet Malaysia Market demands. Topics involved the market segments of Malaysia, registration requirements and unique market information of Malaysia market.

E-labelling No. 2-2025 committee meeting
On 15 May 2025, MAPS participated in the E-labelling Bil 2-2025 meeting with NPRA , MOPI and PhAMA. This meeting was chaired by Pn Rosliza Binti Lajis, Acting Director of NPRA.
Preliminary Findings on acceptance of E-labelling by Dr June Choon, Universiti Technology Mara is as below:
- Multistakeholder study involving Industry, Regulator, HCPs, Public.
- Strong support for E-labelling due to environment impact, ease of updates, improvement in information accessibility.
- HCPs (30% DR, 10% Pharmacists, 50% Nurses) - 80% open or willing to adopt. However, 75% have not used before.
- Public - 92% willing to use if available. However, 69% have not used it. only 46% heard of it before.
- Quest 3+ is the preferred host as neutrality and centralization governed by NPRA.
- Lack of Asean Harmonization. Difficult to share pack.
- Challenges- workflow changes within the company.
- Benefits- Faster dissemination of updated information.
- Concerns - Special populations. Patient resistance to technology, technological barriers. Caregiver empowerments are important here.
- Remain voluntary for the time being.
- Extend to OTC in July 2025.
- Final survey report by July 2026, to be submitted to DCA by Dec 2026.
The presentation is available here.
|  Meeting with Ministry of Health on Medicines Priority List
The Proecurement and privatisation Department of the MOH held an on-line engagement session with Malaysian Organisation of Pharmaceutical Industries (MOPI) and Malaysian Association of Pharmaceutical Suppliers (MAPS) on the above matter. The MOH wishes for our members and MOPI's to source for a list of prioirty products, which have been distribted to members. Registration of such products may be given fast track registration but all the documents must still be acceptable to the NPRA to qualify.
The meeting was held on 8 May 2025 from 12.30 - 1.00 afternoon chaired by Datuk Mohd Fauzee bin Abd Majid, secretary of the department.
|
Reject Illegal Medicines Roadshow launch
MAPS attended MAJLIS PERASMIAN PROGRAM KEMBARA TOLAK UBAT TIDAK SAH (TOBaTS) ZON TENGAH 2025 on 3 May 2025 at Kuala Selangor. This is the launch event officiated by our Health Minister YB Datuk Seri Dr. Dzulkefly bin Ahmad aimed at educating the public on identifying illegal medicines and to reject such medicines. With this education, not only the safety of the people is improved, but our threat from such products are also addressed.

Pharma Supply Chain Resilience Webinar
On 30 April 2025, MAPS attended a webinar on Pharma Supply Chain resilience: What's changing and how to stay ahead. Presentation slides has been sent to all members via email. Below are some pointers:
Global Trend in Pharmaceutical Supply Chains ( by Mr Devaraj Subramaniam, IQivia consulting group)
- Then & now of the current US administration
-
Potential implications for the pharma supply
-
Pharmaceutical exempted from Tariff currently.
-
Potential implication to pharma supply:
-
Increase Drug Cost (esp. generics to US) and Shortage (esp in US)
-
Rising protectionism & local manufacturing
-
Supply chain rebalancing.
What can local Pharma and Gov do? ( by Ng Yee Theng , IQivia)
- diversify export market
- strengthen regional trade.
- focus on innovation & value.
- engage in diplomatic trade dialogue
- Build resilient local supply chain.
Building Pharma Supply Chain Resilience in Malaysia (BY Dr Sithra Devi , MITI)
- Critical challenge
- Resources (Single source, API imports, Far sourcing)
- Human capital (brain drain, Foreign labour)
- R&D ( Access to financing )
- Logistics ( Port congestion & Increase cost, long delivery cycle. )
- High dependence on imported pharmaceutical + Stockpile (<1 year)= Vulnerability in supply chain.
- Critical success factors
- Manufacture domestically high quality API.
- Geopolitical diversification of pharma supply chain.
- Multiple domestic & foreign manufacturers for each product and its precursors.
- Adequate skilled workers
- Increase in R&D
India's take away- Lessons from Pandemic ( by Ms Archana, Indian Pharmaceutical Alliance IPA)
- IPA exports 80% of the country's pharmaceutical.
- India exports 20% of the global generics.
- However, around 70% API are coming in from China.
- Key industry action during pandemic:
- Fast tract approval
- Emergency authorization of selected molecules
- Virtual SEC meeting
- 3-4 months inventory stock level including API.
- Alternative suppliers esp from Europe.
- Digitalization.
Good Regulatory Practice Workshop on Screening Practices for New Drug Products and Biologics
MAPS attended Good Regulatory Practice Workshop on screening practices for new drug products and biologics on 16 April 2025. Global perspective of regulatory system in Malaysia shown that there's 70% rejection rate for screening. This workshop aimed to help reduce the rejection rate. A high rejection rate is not good for both the industry and NPRA, as both sides would have lost productive time in the screening process.
A new screening package will be implemented w.e.f May 2025 which includes:
- Cover letter & new screening check list to be uploaded to E14 and S10
-
Relevant forms (eg Non clinical GLP certificates E14, FRP E14, POA E12 BE P9 etc)
-
Guidance for industries.
NPRA insists on valid GMP 3 years from date of inspections during screening. The slides will be circulated to members once received.

Annual General Meeting 2025
On 14 April 2025, MAPS held its 14th AGM with 58% quorum. 25 out of 38 members turned up for the AGM. Various issues were discussed mainly on regulatory matters. The board of directors will act upon some of the suggestions given like holding a PV workshop in the coming months. Having 2 additional issues to be discussed with NPRA, namely, to accept CoPP (for export only) and to accept first 3 imported generics as fast track in the dialogue with NPRA in May 2025.

NPRA consultation Medicine shortage reporting system
On 14 April 2025 9am, a consultation invitation from NPRA has been conducted on Medicine shortage reporting system; whereby Sofina, representing MAPS has given the below summary and slides have been sent via email to all members.
- This procedure is created following the shortage of medicine that happened in 2020, and the recent issue of insulin shortage
-
The scope is of all medicine (pharmaceutical product) including vaccines, which contains Poison for human use. (exclude Vet products)
-
Reporting of medicine shortage and medicine discontinuation. PRH is to report 6 months before shortage / discontinuation. For shortage that happened suddenly, it must be reported immediately
-
Reporting is via google form. Once NPRA gets the report, they will inform other PRH with the same active ingredient to get info if the same product is marketed or not. Please refer to slide no 7 for details on the action taken by NPRA once they receive the report
-
Medicine Shortage Database will be published in NPRA website (public domain), with active database, after directive is being issued out. At the moment, access to the Medicine Shortage Database can only be access through the link in the guidance document (which is still in draft)
-
Regulatory action will be taken for companies that breach mandatory reporting obligations. Please refer to slide no 10 on this matter
-
The draft procedure is open for public comments in UPC until 5 May 25
-
It will then be tabled into Jun DCA meeting, and directive will be issued
-
Implementation will be Jul 25 (voluntary) and Q1 2026 for mandatory implementation
Tariff Impact Assessment on Pharmaceuticals and Medical Devices
The Ministry of Health called for an impact on the above matter, chaired by the Deputy Secretary General (Finance), MOH, Dato' Seri Norazman Ayob. The engagement Session was held on the 9th of April 2025 from 10.30-12.30 at the Ministry of Health, Putrajaya. Thanks to Mr Dharshen, the minutes are recorded as follows.
The meeting obtained stakeholder's feedback on the impact of the imposition of tariff's by the USA with on-site participation by
PhAMA, MOPI, MAPS, Malaysian Medical Device Association (MMDA), Association of Malaysian Medical Industries (AMMI), Association of Manufacturers of Malaysian Medical Device (PERANTIM). Among other organisations that joined via on-line engagements was the Malaysian Rubber Glove Manufacturers Association (MARGMA).
Based on the latest published data (2023), total exports of Medical Devices from Malaysia was RM 37 billion (out of which RM 8 billion was the gloves export), and RM 14 billion was exported to the USA.
Hence, the USA is a significant market nearly 40% of the total export market and gloves are a key export (nearly 20% of the total export market).
The AMMI, MMDA & PERANTIM all suggest an increase in cost is likely due to direct costs (increase in export costs due to the tariff ) and indirect costs (as many medical devices exported from the USA require components that are needed to be imported which are subject to tariffs).
As for MOPI, the latest published data (2023) states that total imports of Pharmaceuticals was RM 11 billion whilst the total export market size was RM 2 billion.
MOPI expressed concerns that the tariffs will cause an increase in the price of APIs from countries with a relatively lower tariff rate (for eg Metformin API from Norway) as there will be more demand from the USA to import API and there will be less priority for smaller markets like MY, which may also impact medicine security.
Nevertheless, there will be opportunity to import APIs from higher tariff nations (India ; 26% tariff and China ; 104%) as these manufacturers will consider "dumping" their API to non-US nations but there is a possibility of countries that uses these API's may be subject to increased tariff's from the USA (as the USA may perceive this as a backdoor method of bypassing the tariff's imposed on high tariff nations).
TKSU also shared that the USA's method of imposing reciprocal tariff also takes into account "non-tariff trade barriers" such as the MoH's policy of preferring local manufacturers for tender awards, among other policies.
He suggests that the MoH may need to revisit these policies in order to lower the overall tariffs imposed on MY.
Finally, TKSU also requested for stakeholders to provide feedback to the MoH by Friday at the latest, to be presented to the honourable Prime Minister next week prior to the discussion with the USA to negotiate the tariff's.
TKSU also reiterated the overall need for the MoH to reduce costs for drug & device procurements, further engagements to align MAPS interest for tender procurements should be made after the tariff's discussion are concluded.

Monash University Pharmaceutical Science Advisory Group 1st 2025 meeting
The advisory group met online for its first meeting for the year 2025 on the 7th of April 2025. The advisory group meets twice a year. MAPS members are advised that there are opportunities to hire interns who are pursuing the Master's degree for periods of 4 to 12 weeks. These interns can undertake projects helpful to member companies. Such opporunities will be shared with members when the time arises.
Public Accounts Committee meeting with Industry
The Parliamentary Public Accounts Committee summonned the pharmaceutical industry to a proceeding as witnesses on the subject of the healthcare insurance premium increase, hospital charges and the impact on public health, under the Ministry of Finance, Ministry of Health and Bank Negara Malaysia.
The proceeding was held on 6th March 2025 before the PAC, chaired by Datuk Wira Hajah Mas Ermieyati binti Haji Samsudin together with several members of Parliament who are PAC members. The witnesses called were from MAPS, Phama, MOPI and MPS. MAPS was represented by the President. 
Good Governance in Medicines modules discussion
A discussion was held to develop GGM modules for private sector chaired by Pn Nur' Ain Shuhaila binti Shohaimi, deputy Director, Bahagian Dasar & Perancangan Strategik Farmasi , PSP on 24 Feb 2025. So far MOH has several modules for public sector, both for Provisionally registered pharmacist (PRP) & fully registered pharmacist (FRP):
- Modul pengenalan (Introduction Module)
- Modul tadbir Urus Ubat-ubatan yang baik(Good Governance fo Medicine Module)
- Modul Integriti & Etika(Integrity & Ethics Module)
- Modul Garis panduan keselamatan & pengendalian stok di fasiliti kesihatan & kes sebenar. (Security Guidelines & stock handling in health facilities & actual cases Module)
- Modul tindakan tatatertib & hukuman(Disciplinary actions & penalties Module)
Moving forward, task force to be formed and MOH will share the slides for industry feedback first; followed by a brainstorming session at Dewan Serai Wangi.
Public engagement by Public Accounts Committee on the Insurance Premium Hike and Private Hospital Charges
Medical insurance premiums rising by 40 to 70% has caused some public outcry. Read about it here. The Parliamentary Public Accounts Committee (PAC) has started a series of engagements to investigate this issue. The first was conducted in Penang last week, and the second public engagement was held at the Parliament on 21 Feb 2025.
MAPS president attended this meeting which was chaired by YB Sim Tze Tzin together with several other members of Parliament with representatives from the Bank Negara Malaysia and many other government agencies. Over 570 persons attended, with 51 persons from all walks of life who spoke at this session that was held from 9 am to 12.30 pm.
While the President spoke on the general role of pricing and generics in managing healthcare costs, the PAC has extended an invitation for a closed door session in March. We hope to be able to present a more comprehensive position when we attend the session involving the 3 industry associations. Members are encouraged to provide feedback to the secretariat by end of February, to allow time for our views to be fully captured when we meet the PAC.

Technical Committee meeting for DUNas 2025 component 2: Quality , Safety & Efficacy
MAPS attended Technical Committee DUNAS Component 2: Quality , Safety & Efficacy which was chaired by Pn Rosliza Lajis, Deputy Director, Centre of product & cosmetic evaluation on 19 Feb 2025. Below are few key points:
- For NPRA to reach WHO maturity level 3, requires 65 institutional development plans (IDP). 56 IDP on going, 19 requires amendment to Sales of Drug Acts 1952. Amendment to SODA already published in UPC.
- For NPRA to be recognised as ICH members, requires funding. Tabung Amanah L720 set up to receive donation from associations.
- Cell Gene Therapy registration guidelines to be published in Q2 2025. Currently only 1 product approved. 6 GMP inspections , 1 GLP and 1 CTIL/ CTX.
- First in human FIH clinical trials. 6 applications received to conduct FIH study locally.
- NPRA as statutory body idea rejected by MOF. Instead to pursue as Badan Ber-autonomy.
- Online sales of illegal & falsified medicines a total of 11527 cases in 2024 leads to related advertisement removal in local e-marketplace.
- Enforcement to reduce sales of unregistered products reduction by 60% achievement.
- Pharmaceutical Tract and trace (PTTS) to be in 1st phase voluntarily basis for all poison target to start in 2027. July 2025-June 2026 to set up repository system. The company that wins the tender of hologram will be task to set up this repository as well.
- PV inspections- 9 companies volunteered to be inspected. 2 companies from MAPS passed the inspections. Change of Acts requires to involve mandatory PV.
- 5 test methods developed locally by NPRA to test vaccines.
- FRP facilitated registration pathway. NCE:9, Biologics: 6 Generics: 3 approved under this pathway in 2024.

E-Labelling Committee Meeting no. 1/2025
E-Labelling Committee Meeting no. 1/2025 was held on 18 Feb 2025 Chaired by Dr Azuana Ramli, Director of NPRA. MAPS represented by Tharishini, Hui Wei, Ee Lin , Phuvi and Siew Mei. Below are some key points:
- Pilot Study on E-labelling second round of data analysis on going and presentation will be held in Mar/April 25.
- Video of E-labelling will be translated from English to BM. To display at NPRA Facebook page, NPRA website consumer section.
- 30 biologics, 261 generics and 96 NCEs with a total of 387 products approved for e-labelling.
- To open to non-association members by July 2025.
- To expand to OTC by July 2025.
- Quest 3+ down time in Dec due to hardware issues, measures have been undertaken to prevent such occurrence. Quest 5 will be cloud based.
- To move "Technical evaluation summary" to another landing page. It is a requirement to publish this for public access.
- NPRA to discuss internally if bundling of variation is allowed.
- Wait for study outcome, to decide if DHCP letter is still needed.
- Cannot share pack with other countries which implement E-labelling due to different QR code used. Future to have 1 international pack, with 1 QR code. Geo-fencing at backend, so that each country will direct to different landing pages. Possible to harmonized within ASEAN. Ideally, ASEAN regulator to accept MY e-labelling.

Technical Committee meeting for DUNas 2025 component 5: Partnership and collaboration for the healthcare industry
Technical committee for Dunas component 5: Partnership and collaboration for the healthcare industry was held on 17 Feb 2025, chaired by Dr Azuana Ramli, Director of NPRA.
Below are a few key points:
- Disposal of medicines by consumer via community pharmacists MY Medisafe was launched on 25 Sept 2024.
- Pilot project of E-prescription from KKM facilities to Community pharmacies was started on 2 Dec 2024 within Penang state, with 4 KKM facilities (Hosp P Pinang, Hosp B Mertajam, KK Jln Perak, KK Seberang Jaya) involved with 55 community pharmacies.
- Based on study, Estimating Community Pharmacists Service Fee, a proposal for service fee of RM6 per repeat prescription was made. RM1 will be paid to ProtectHealth as service purchaser for the purpose of service management of Ubat%40Komuniti which includes training and monitoring of services given by community pharmacies involved.
- E-pharmacy task force to be formed by MPS - enforcement.
- Pharmacy Research Priorities Malaysia (2nd edition) was launched by YBMK on 19 Aug 2024. 11 studies published which is collaboration between private-public sector. Mainly by the universities.
- Regional collaboration strategy for ASEAN Drug Security & Self Reliance (ADSSR) Plan of action to be postponed to May 2025.
- Tabung Amanah L720 was set up to receive donation from associations or 3rd parties for the purpose of NPRA achieving ICH accreditation. The fund will be used for training purposes.
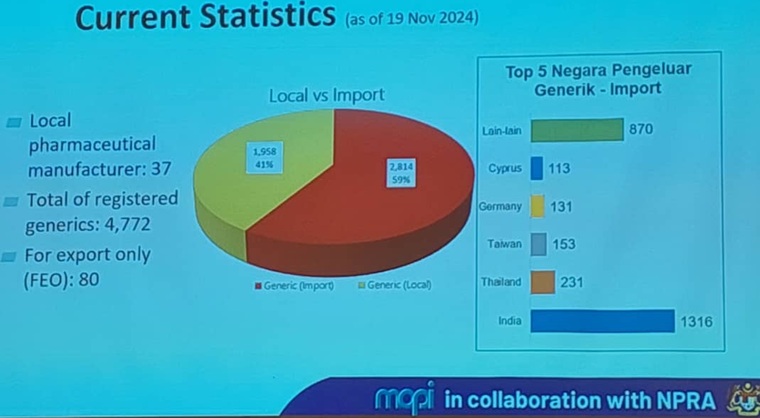
Technical Committee meeting for DUNas 2025 component 3: ACCESS TO MEDICINES
On 7 Feb 2025, JKT Dunas component 3 Access to Medicines was held and chaired by Pn Wan Noraimi, Director of Pharmacy Practise and Development. This is to update all stakeholder of the direction and status of the implementation. An executive summary is available here.

Technical Committee meeting for DUNas 2025 component 1: GOVERNANCE IN MEDICINES
On 4 Feb 2025, JKT Dunas component 1 Good Governance of Medicines was held and chaired by Puan Siti Aisah binti Bahari, Director of Policy and Strategic Planning, PPP for 2022-2026. This is to update all stakeholder of the direction and status of the implementation. Revision of GGM training framework achieved with 32 trainers trained and 386 participants attended the workshop. Guidelines on MY CPD KKM to be published by early 2025. 1115 pharmacists were granted credentialling within MOH.

Engagement session on DG's Directive regarding Poisons Act
On 8 Jan 2025, PSP Enforcement division conducted a dialogue with all stakeholders including MAPS to inform of upcoming amendment in Poisons Act 1952 to:
- increase an additional of 7 antibiotics [ Apramycin, Bacitracin, Enramycin, Tiamulin, Trimethoprim, Virginiamycin and zinc bacitracin] under prohibition to sell wholesale for use as food products promoting growth or prophylaxis of animal use by any licensed pharmacists.
- prohibit to import and /or sell nicotine for manufacturing of cigarette productss by any licensed pharmacists.
These directives are to contain antimicrobial resistance and also to safeguard the professionalism of pharmacist in not promoting cigarette. The prohibition on nicotine does not extend to products for tobacco cessation, eg chewing gum and patches.

Good Submission Practice Workshop
On 20 to 21 November 2024, NPRA generics division held a good submission practice workshop. Slides deck has been emailed to everyone on 19 Dec 2024. Good submission practice would ensure that both sides, ie the regulators and companies get the approval quicker. 3 points to take note:
- Dossier can be submitted for evaluation after getting GMP inspection date only.
- For study specific BE centre inspection, dossier can only be submitted after 20k payment been made to NPRA.
- Q2 2025 NPRA will require Quality Overall Summary (QOS) to be submitted in the relevant section.
MAPS Dinner 2024 Photos
Please click on this image below to go to the link page.

Award: Honouring Pharmaceutical Services Programme (PSP)'s Leadership in Crisis Management - Pandemic Resilience
During MAPS dinner on the 13th anniversary, MAPS honoured the PSP Leadership in Crisis Management during the pandemic for Pandemic Resilience. The text of the citation is here.

Photos of the dinner will be uploaded soon.
Stakeholders Engagement session with NPRA
A dialogue has been conducted between NPRA and all Stakeholders on 15 Nov 2024, regarding the below changes:
1st Topic: Risk-based Product Quality Monitoring (Risk-based PQM)
Intro: Currently the PMS is done for each product once for every 5 years life cycle. Initially there are 4 steps (IMG_2031). With this risk-based approach, there are 8 steps (IMG_2033). IMG_2033 are the steps involving PRH.
- This will involve all products except cosmetics
- Targeted to implement in Q3 2026
- Will start the process of selecting the products for PMS based on risk-based assessment in 2025-2026 Q1/Q2. During this period, NPRA will email the PRH the list of products. PRH to ensure complete and updated documents for sections E12, P5.1 and P5.2 are uploaded to Quest 3+ according to the list of products as NPRA will use those documents to test the samples of the selected products. Uploading in Quest 3+ will be done through data cleaning module.
- Directive for this new type of PMS will be out next month.
2nd Topic: Vaccine : Method Transfer and Method Verification
- Currently Method Verification is done for locally manufactured vaccines. For imported vaccines, only evaluation of documents is conducted.
- As NPRA had a finding during their WHO audit last year, this initiative is taken as a measure to close the finding.
- NPRA will be building a new laboratory facility in 2027 to carry out method transfer for all vaccines, including imported vaccines.
- In this Method Transfer and Method Verification, manufacturers are to transfer the method to NPRA and NPRA will use the method to do the testing in the new lab.
- Targeted to implement in 2027 when the new facility is ready. NPRA will start with testing according to priority. However, "exclude" does not mean forever but just for the time being.
- For vaccines registered in 2025 and 2026, method verification will be done in 2027. NPRA will provide list.
- PRH responsibilities are listed out in IMG_2056.
- Full cooperation from the industry is needed because if the method verification fails, then the product will be rejected.
- All cost for method transfer and method verification is to be borne by the PRH.
- Method verification will only be done once for the whole product’s life cycle.
- Lot release is not method verification. Hence, lot release still needs to be done. But for imported lot release, lot release testing not done.
- NPRA will update if there are any changes in the future.
3rd Topic: API requirements in natural products registration
2 Batches of COA for API of natural products required to be uploaded to Quest system. And upon receival of raw material, testing needs to be conducted according to monograph specification. Implementation date is voluntary from 2025 until Dec 2027; Mandatory from 1 Jan 2028.

Insights into New Guidelines: MOH Medicine Formulary Dossier Submission and Medicine Access Scheme
MAPS attended a Workshop entitled "Insights into New Guidelines: MOH Medicine Formulary Dossier Submission and Medicine Access Scheme in Public Health Facilities for 2024" by Pharmaceutical Services Programme, Ministry of Health (MOH) on 5 November 2024 (Tuesday) from 9 AM to 5 PM. 4 slide decks have been circulated to members' CEOs via emails on 15 Nov 2024.
Site Specific Stability Study meeting
An online discussion on site specific stability study had been conducted on 5 November 2024 between NPRA with 3 associations MAPS, MOPI & PhAMA. NPRA has taken MAPS proposal to adhere to Asean guidelines and to reduce the requirement when multiple API manufacturers are proposed for the Finished product to a commitment to conduct finished product stability study for 1 production batch only instead of 2 or 3 production batches. The amendment will be enforced in Jan - July 2025.
Monash University Pharmaceutical Science Advisory Group meeting
| Monash University Pharmaceutical Science Advisory Group meeting met online on 21 October 2024. The terms of reference for the group
is to provide expert advice and professional guidance on matters and needs relating to the Pharmaceutical Science Programs (the Programs) at Monash University Malaysia (MUM).
The Group will undertake the following activities:
- Provide an avenue of communication between the University and the pharmaceutical industry, advocating for advancement in pharmaceutical science and the industry.
- Provide external and professional feedback on the suitability of the undergraduate and postgraduate Pharmaceutical Science curricula to meet the current and emerging needs of the industry.
- Advise on the educational and training needs and outcomes of the professional internships, including needs related to supervisors.
- Assist with any relevant course accreditation and recognition matters.
- Act as a sounding board to assist the development of new ideas and initiatives in support of quality improvements.
With this program, the course is targeted towards an industry lean, which will provide potential talents who are much more inclined to the challenges of the industry. |  |
Farewell Lunch for NPRA Director
On 4 Oct 2024, MAPS Regulatory Affairs subcommittee had a farewell lunch with Pn Rosilawati , Pengarah of NPRA to celebrate her retirement and to thank her for the support she rendered to MAPS all these years while she was in service.

View Archived News
June 2012 and earlier
July 2012 - Dec 2012
Jan 2013 - June 2013
Jul 2013 - June 2014
Jul 2014 - Nov 2014
Dec 2014 - Jul 2016
Aug 2016 - Dec 2017
Jan 2018 - Dec 2020
Jan 2021 - Dec 2022
Jan 2023 - Oct 2023
Nov 2023 - Sep 2024
|
| Content on this site is copyrighted to the Malaysian Association of Pharmaceutical Suppliers.
|
|
